Johnson–Corey–Chaykovsky fluorocyclopropanation of double activated alkenes: scope and limitations†
Abstract
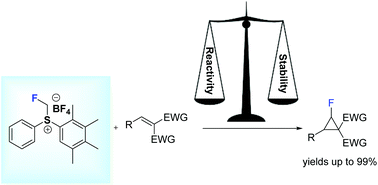
Johnson–Corey–Chaykovsky fluorocyclopropanation of double activated alkenes utilizing S-monofluoromethyl-S-phenyl-2,3,4,5-tetramethylphenylsulfonium tetrafluoroborate is an efficient approach to obtain a range of monofluorocyclopropane derivatives. So far, fluoromethylsulfonium salts have displayed the broadest scope for direct fluoromethylene transfer. In contrast to more commonly used fluorohalomethanes or freon derivatives, diarylfluoromethylsulfonium salts are bench stable, easy-to use reagents useful for the direct transfer of a fluoromethylene group to alkenes giving access to the challenging products – fluorocyclopropane derivatives. Interplay between the reactivity of the starting materials and stability of the fluorocyclopropanes formed determines the outcome of the process.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...