Carbon supported noble metal nanoparticles as efficient catalysts for electrochemical water splitting†
Abstract
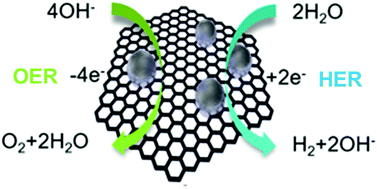
Due to an increasing requirement of clean and sustainable hydrogen energy economy, it is significant to develop new highly effective catalysts for electrochemical water splitting. In alkaline electrolyte, Platinum (Pt) shows a much slower hydrogen evolution reaction (HER) kinetics relative to acidic condition. Here, we show a versatile synthetic approach for combining different noble metals, such as Rhodium (Rh), RhPt and Pt nanoparticles, with carbon forming noble metal nanoparticles/nanocarbon composites, denoted as Rh(nP)/nC, RhPt(nP)/nC and Pt(nP)/nC, respectively. It was found that in alkaline media these composites exhibited higher performance for the HER than the commercial Pt/C. In particular, Rh(nP)/nC displayed a small overpotential of 44 mV at a current density of 5 mA cm−2 and a low Tafel slope of 50 mV dec−1. Meanwhile, it also showed a comparable activity for the oxygen evolution reaction (OER) to the benchmarking catalyst RuO2. The superior HER and OER performance benefits from the very small size of nanoparticles and synergy between carbon support and nanoparticles.
- This article is part of the themed collection: Chemistry of 2D materials: graphene and beyond


 Please wait while we load your content...
Please wait while we load your content...