The role of cations in hydrothermal synthesis of nonlinear optical sodium niobate nanocrystals†
Abstract
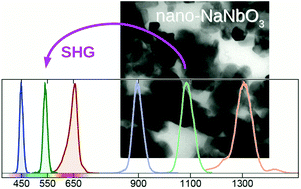
The usability of the alkali niobates with their ferroelectric and photorefractive properties could be expanded if the development of synthesis methods would allow to obtain small, preferably monodispersed, crystals in the sub-μm to nanometer regime. Of all the possible synthesis methods, the most reliable is currently hydrothermal synthesis to generate small crystallite sizes of these materials. Although the products of sodium niobate are polydisperse and partially agglomerated, they show a significant SHG signal that is unexpectedly comparable to that of potassium niobate. A view on the hydrothermal synthesis of sodium niobate reveals that the incorporation of cations in the crystalline lattice of the niobium educt plays a part in the formation of the product. The occurrence of distinct different phases, as in the case of potassium niobate, is not observed. Instead, it is shown that a clear assignment of the crystalline phase cannot be made here. This indicates that crystallization of the alkali niobates in hydrothermal synthesis depends on the stoichiometry, the niobium starting material and the cation used.


 Please wait while we load your content...
Please wait while we load your content...