The enzyme-induced formation of iron hybrid nanostructures with different morphologies†
Abstract
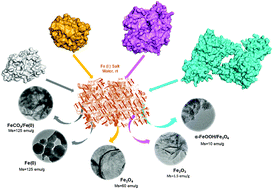
A new synthesis method for tailor-made iron-hybrid nanoparticles has been carried out for the first time using enzymes, which directly induce the formation of inorganic iron species. The role of the protein was critical for the formation and morphology of the iron nanostructures and, depending on the enzyme, by simple mixing with ammonium iron(II) sulfate at room temperature and under air, it was possible to obtain, for the first time, well stabilized superparamagnetic iron and iron oxide nanorods, nanosheets and nanorings or even completely amorphous non-magnetic iron structures in the protein network. These iron nanostructure-enzyme hybrids showed excellent results as heterogeneous catalysts in organic chemistry (chemoselective hydrogenation and C–C bonding formation) and environmental remediation processes.


 Please wait while we load your content...
Please wait while we load your content...