Antitumor effects of novel nickel–hydrazone complexes in lung cancer cells†
Abstract
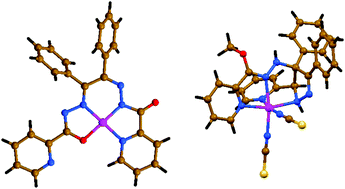
In this work we have synthesized and characterized two new mononuclear nickel(II) complexes [NiLI]·CH3CN (1·CH3CN) and [Ni(H2LII)(NCS)2]·0.5H2O (2·0.5H2O), fabricated from a mixture of Ni(NO3)2 and KNCS with N′,N′′′-(1,2-diphenylethane-1,2-diylidene)di(picolinohydrazide) (H2LI) and 1,2-diphenyl-1,2-bis(((pyridin-2-yl)methylene)hydrazono)ethane (Lig), of which the latter one was transformed in situ into methyl-N-(3,4-diphenyl-5-(pyridin-2-yl)-4,5-dihydro-1H-pyrazol-4-yl)picolinohydrazonate (H2LII) upon coordination to the metal center under synthetic conditions in methanol. The nickel(II) atom in the structure of complex 1 is tetracoordinated and in a N3O geometry, formed by one doubly deprotonated ligand LI, linked through the pyridyl nitrogen atom, one of the amide nitrogen atoms, one of the imine nitrogen atoms and one of the carbonyl oxygen atoms, yielding an almost perfect square-planar coordination environment. The crystal packing of 1·CH3CN is dictated by a set of weak non-covalent interactions, namely C–H⋯O, C–H⋯π and π⋯π stacking interactions. The asymmetric unit of complex 2·0.5H2O contains two crystallographically independent complex molecules [Ni(H2LII)(NCS)2], namely 2-I and 2-II, which are geometrically very similar. The metal atoms are six-coordinated and in a N6 geometry, formed by one neutral ligand H2LII, linked through two pyridyl nitrogen atoms, one amine nitrogen atom from the pyrazole ring, one imidate nitrogen and two NCS− nitrogen atoms, yielding a distorted octahedral coordination environment. The crystal packing of 2·CH3CN is mainly dictated by intermolecular N–H⋯N, N–H⋯S and O–H⋯S hydrogen bonds, yielding a 3D framework, which is further stabilized by intermolecular π⋯π stacking interactions. From the topological point of view, the hydrogen bonded 3D framework of 2·0.5H2O reveals a four-connected uninodal crb/BCT; 4/4/t5; sqc184 topology. In in vitro experiments, both complexes showed dose dependent cytotoxicity and killed A549 lung cancer cells via an apoptotic pathway. Both complexes also reduced the expression of Snail2, leading to an increase in E-cadherin expression and inhibiting the motility of the cells. The complexes were determined as potential antitumor agents.


 Please wait while we load your content...
Please wait while we load your content...