Mitochondria-targeted delivery and light controlled release of iron prodrug and CO to enhance cancer therapy by ferroptosis†
Abstract
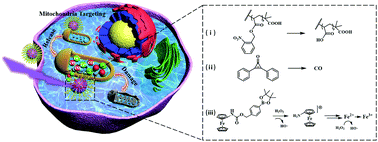
Mitochondrial malfunction is considered to be a decisive signal of apoptosis. It would be a promising strategy to target mitochondria in cancer cells to generate reactive oxygen species (ROS), thus directly inducing mitochondrial damage. We herein reported a mitochondria-targeted, photo-responsive polymer (Mito-PNBE), which can self-assemble into nanoparticles (Fe–CO@Mito-PNBE) encapsulated with diphenylcyclopropenone (light-responsive CO prodrugs) and aminoferrocene-based prodrugs via hydrophobic interactions. Upon UV-irradiation, the rapid release of CO and aminoferrocene-based prodrugs caused by disassembly was observed. On one hand, the released carbon monoxide in mitochondria could enhance ROS generation and accelerate oxidative metabolism. On the other hand, the aminoferrocene-based prodrugs will release Fe3+/Fe2+ ions in the tumor microenvironment, thus triggering the Fenton reaction, which generates more ROS and damages the mitochondria. Thus, the synergistic effect of the two drugs produces enough amounts of ROS in the mitochondria, leading to mitochondrial collapse with an enhanced cancer therapeutic effect. This multifunctional platform has potential in precision cancer therapy.


 Please wait while we load your content...
Please wait while we load your content...