Evaluation of the supramolecular interaction of Congo red with cucurbiturils using mass spectrometry and spectroscopic methods†
Abstract
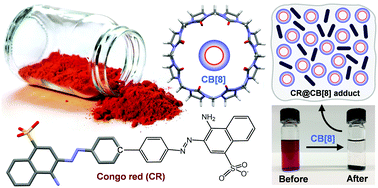
The ability of cucurbit[n]urils (CB[n]) to decolourise aqueous solutions of the azo dye Congo red (CR) was described more than a century ago alongside the first synthesis of CB[n]. No subsequent studies of the nature of the physical interactions have been reported despite the interest in using CB[n] as adsorbents for the removal of CR and related organic dyes from wastewaters. In the present work the supramolecular interaction between CB[n] (n = 7, 8) and CR was studied by electrospray ionisation mass spectrometry (ESI-MS), 1H NMR, and solid-state characterisation of isolated complexes. Under positive ESI, the formation of host–guest complexes in the gas phase was not observed, suggesting that CR anions do not interact with the portals and the nonpolar inner cavity of the CB[n] molecules. Conversely, under negative ESI, 1 : 1 and higher order (1 : 2, 2 : 1, 3 : 1 and 2 : 2) CR : CB[7] and CR : CB[8] adducts were detected, which is attributed to interaction between CR and the outer surface hydrogens of CB[n]. Solid-state supramolecular adducts between CB[n] and CR were isolated from aqueous media under either ambient conditions, giving structures denoted as CR@CB[n](RT), or hydrothermal (100 °C) conditions, giving structures denoted as CR@CB[n](100). The adducts were characterised by elemental and thermogravimetric analyses (TGA), powder X-ray diffraction (PXRD), and spectroscopic methods (FT-IR, FT-Raman, 13C{1H} CP MAS NMR, UV/vis and near-IR absorption, fluorescence excitation and emission). The interaction conditions and acid content of the starting CB[n] influenced the protonation state of CR molecules. CR@CB[7](RT) contained only unprotonated CR, while CR@CB[8](100) contained exclusively protonated CR (ammonium and azonium/quinoid structures). Other adducts contained mixtures of protonated/unprotonated forms.


 Please wait while we load your content...
Please wait while we load your content...