Molybdenum oxynitride nanoparticles on nitrogen-doped CNT architectures for the oxygen evolution reaction†
Abstract
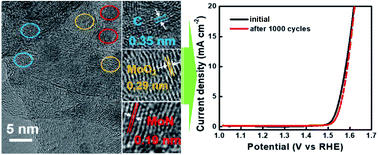
Transition metal-based electrocatalysts are considered the potential alternative to noble metal-based ones owing to their comparable electrocatalytic properties, durability, and low cost for the oxygen evolution reaction (OER). Herein, we report the partial nitridation of molybdenum oxide nanoparticles anchored on nitrogen-doped carbon nanotube (Mo–N-CNT) architectures for a highly active OER electrocatalyst. The molybdenum oxynitride nanoparticles are uniformly distributed on the surface of hierarchical N-CNT architectures, where nitrogen heteroatoms are incorporated through the thermal decomposition of carbon nitride. The modified surface chemistry can boost the electrocatalytic activity of Mo–N-CNT to show improved electrochemical behaviours for OER operation. The Mo–N-CNT achieves a current density of 10 mA cm−2 with an overpotential of 344 mV, Tafel slope of 64 mV dec−1, and current density retention of 79% during the oxidation in an alkaline electrolyte for 80 h. The enhanced electrocatalytic performance of Mo–N-CNT is attributed to the hierarchical N-CNT structure and nitridation of Mo oxides.


 Please wait while we load your content...
Please wait while we load your content...