The correlation between phase transition and photoluminescence properties of CsPbX3 (X = Cl, Br, I) perovskite nanocrystals†
Abstract
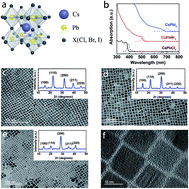
We report a correlation between the structural phase transition of CsPbX3 (X = Cl, Br, I) nanocrystals (NCs) and their temperature-dependent steady-state photoluminescence (PL) and time-resolved PL (TRPL). In contrast to CsPbBr3 and CsPbI3 NCs which exhibited a continuous blue-shift in their band gap with increasing temperature, the CsPbCl3 exhibited a blue shift until ∼193 K, followed by a red shift until room temperature. We attribute this change from a blue to a red shift to a structural phase transition in CsPbCl3, which also manifested in the temperature dependent TRPL. This pronounced phase transition in CsPbCl3 NCs is probably due to the condensation of its vibrational modes at low temperature, and the presence of the weak quantum confinement effect. Notably, the exciton recombination lifetimes showed a similar reverse trend due to the phase transition in CsPbCl3, which has not been reported previously.


 Please wait while we load your content...
Please wait while we load your content...