Pyrazolyl-pyrimidones inhibit the function of human solute carrier protein SLC11A2 (hDMT1) by metal chelation†
Abstract
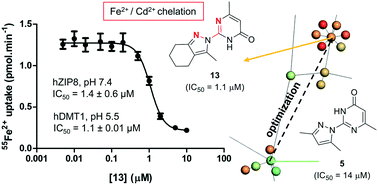
Solute carrier proteins (SLCs) control fluxes of ions and molecules across biological membranes and represent an emerging class of drug targets. SLC11A2 (hDMT1) mediates intestinal iron uptake and its inhibition might be used to treat iron overload diseases such as hereditary hemochromatosis. Here we report a micromolar (IC50 = 1.1 μM) pyrazolyl-pyrimidone inhibitor of radiolabeled iron uptake in hDMT1 overexpressing HEK293 cells acting by a non-competitive mechanism, which however does not affect the electrophysiological properties of the transporter. Isothermal titration calorimetry, competition with calcein, induced precipitation of radioactive iron and cross inhibition of the unrelated iron transporter SLC39A8 (hZIP8) indicate that inhibition is mediated by metal chelation. Mapping the chemical space of thousands of pyrazolo-pyrimidones and similar 2,2′-diazabiaryls in ChEMBL suggests that their reported activities might partly reflect metal chelation. Such metal chelating groups are not listed in pan-assay interference compounds (PAINS) but should be checked when addressing SLCs.


 Please wait while we load your content...
Please wait while we load your content...