Development of tuneable green-to-red emitting transparent film based on Nafion with TbIII/EuIII β-diketonate complexes modulated by pH and proton flow†
Abstract
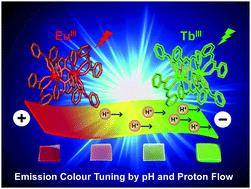
Nafion membranes are recognised as cation exchangers and excellent proton conductors. Emissive LnIII-β-diketonate complexes, [LnIII2(PBA)6] (Ln = Eu, Tb; HPBA = N-(2-pyridinyl)benzoylacetamide), were incorporated into a transparent Nafion film. Upon incorporation, reversible emission by the green light emitter [TbIII2(PBA)6] and red light emitter [EuIII2(PBA)6] could be controlled through pH adjustment. [TbIII2(PBA)6] emitted only under acidic conditions, while [EuIII2(PBA)6] emitted only under basic conditions. Taking advantage of Nafion's high proton conductivity brought about dynamic changes in the wavelengths emitted by the film. When an in-plane external voltage was applied to the film, red wavelength emission appeared to migrate toward the negative electrode. Owing to the proton concentration gradient induced by the electric field, emission by EuIII at 615 nm was enhanced near the positive electrode, whereas proton deficiency suppressed emission by [TbIII2(PBA)6]. Tuneable emission in the transparent Nafion system enhances its potential for use as an energy-saving material.


 Please wait while we load your content...
Please wait while we load your content...