An automated microfluidic system for efficient capture of rare cells and rapid flow-free stimulation†
Abstract
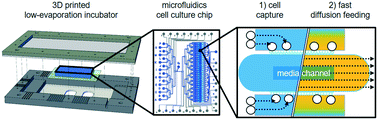
Cell fates are controlled by environmental stimuli that rapidly change the activity of intracellular signaling. Studying these processes requires rapid manipulations of micro-environmental conditions while continuously observing single cells over long periods of time. Current microfluidic devices are unable to simultaneously i) efficiently capture and concentrate rare cells, ii) conduct automated rapid media exchanges via diffusion without displacing non-adherent cells, and iii) allow sensitive high-throughput long-term time-lapse microscopy. Hematopoietic stem and progenitor cells pose a particular challenge for these types of experiments as they are impossible to obtain in very large numbers and are displaced by the fluid flow usually used to change culture media, thus preventing cell tracking. Here, we developed a programmable automated system composed of a novel microfluidic device for efficient capture of rare cells in independently addressable culture chambers, a custom incubation system, and user-friendly control software. The chip's culture chambers are optimized for efficient and sensitive fluorescence microscopy and their media can be individually and quickly changed by diffusion without non-adherent cell displacement. The chip allows efficient capture, stimulation, and sensitive high-frequency time-lapse observation of rare and sensitive murine and human primary hematopoietic stem cells. Our 3D-printed humidification and incubation system minimizes gas consumption, facilitates chip setup, and maintains stable humidity and gas composition during long-term cell culture. This approach now enables the required continuous long-term single-cell quantification of rare non-adherent cells with rapid environmental manipulations, e.g. of rapid signaling dynamics and the later stem cell fate choices they control.


 Please wait while we load your content...
Please wait while we load your content...