The effect of pH and hydrogen bond donor on the dissolution of metal oxides in deep eutectic solvents†
Abstract
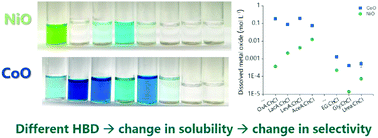
The dissolution behaviour of a series of d- and p-block metal oxides was investigated in deep eutectic solvents, DES, to determine the effect of pH and hydrogen bond donor (HBD) coordination strength on their solubility. The solubility of metal oxides was found to be increased by a higher proton activity in DESs composed of poorly complexing HBDs, due to the ability of H+ to act as an oxygen acceptor. However, the employment of HBDs that have stronger complexing abilities was proven to have a greater effect on the solubility. The strongly coordinating HBDs increased metal oxide solubility via surface complexation reactions followed by ligand exchange for chloride in the bulk solvent. Differing selectivity for leaching of metal oxides was demonstrated, with solubility shown to be broadly dependent on lattice energy and Gibbs energy of formation of the metal oxide, while dissolution kinetics for metal oxides are shown to vary significantly. The results provide pathways to separation of metal oxides by dissolution in DES.


 Please wait while we load your content...
Please wait while we load your content...