Using iron sulphate to form both n-type and p-type pseudo-thermoelectrics: non-hazardous and ‘second life’ thermogalvanic cells†
Abstract
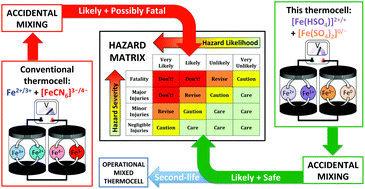
Thermogalvanic cells can act like ‘liquid thermoelectrics’ to convert a thermal energy gradient to electrical energy. Such cells are typically combined electrically in-series in devices to boost the output voltage (as thermocells). However, the typical system involves a potentially fatal combination of inherently acidic or acidified Fe2+/3+ and [Fe(CN)6]3−/4− electrolytes; mixing and heating is expected to trigger extremely toxic HCN gas release. Here we demonstrate that benign aqueous iron(II/III) sulphate can be combined with equally benign sodium sulphate and sodium hydrogen sulphate; the first leads to an [Fe(SO4)2]−/2− thermocell (Seebeck coefficient, Se = −0.4 mV K−1), and the second to a thermocell with intermediate [FeSO4]0/+ and [Fe(HSO4)]+/2+ character (Se = +0.57 mV K−1). Their fundamental thermoelectrochemistry was explored, and their speciation elucidated. It was demonstrated that these can be utilised electrically in-parallel and in-series in thermogalvanic devices. When connected electrically in-series the thermocells presented here displayed temperature-dependent open circuit potentials only ca. one-third that typically reported for the ‘conventional’ combination of Fe2+/3+- and [Fe(CN)6]3−/4−-based thermocells (0.8 mV K−1vs. ca. 3 mV K−1, respectively). However, whereas the latter thermocells cannot be safely mixed, when the iron-sulphate cells were ‘accidently’ mixed they safely form a mixed thermocell electrolyte (Se = +0.19 mV K−1), enabling a ‘second life’ of both the electrolyte and thermocell devices. This novel ‘all-iron sulphate’ thermocell was compared against the typically employed Fe2+/3+ and [Fe(CN)6]3−/4− combination using the 12 principles of green chemistry and of green engineering, further demonstrating the inherent sustainability, safety and ‘green’ credentials of this system (but not yet efficiency). This work demonstrates how functionality and complexity can be introduced in a safe manner, while also preventing potential accidents and enabling new ‘end-of-life’ opportunities.
- This article is part of the themed collection: 2020 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...