Two-step conversion of Kraft lignin to nylon precursors under mild conditions†
Abstract
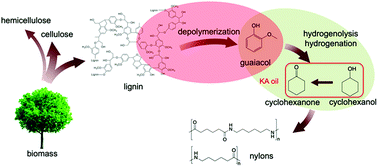
This study explores the valorization of Kraft lignin by conversion into nylon precursors in a two-step process. First, lignin was depolymerized in dilute alkaline aqueous solution under atmospheric N2 at 200 °C to give guaiacol with high selectivity (>80%) with a total monomer production of 13% based on lignin input. Solution and solid state NMR analyses and reactions of model compounds indicated that depolymerization took place via cleavage of β-O-4 bonds in lignin. In the second step, lignin-derived guaiacol was selectively converted to the nylon precursors cyclohexanol/cyclohexanone (KA oil) using Ru/C catalyst under 1 bar H2 and 150 °C. This two-step process constitutes a low-temperature and low-pressure pathway for producing value-added chemicals from lignin using water as the reaction solvent.


 Please wait while we load your content...
Please wait while we load your content...