Covalent triazine framework/carbon nanotube hybrids enabling selective reduction of CO2 to CO at low overpotential†
Abstract
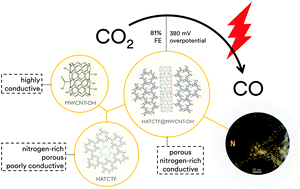
Electrochemical reduction of CO2 provides a way to generate base chemicals from an abundant C1-source under mild conditions, whilst at the same time mitigating CO2 emissions. In this work, a novel class of tailorable, porous electrocatalysts for this process is proposed. Covalent triazine frameworks (CTFs) are grown in situ onto functionalized multiwalled carbon nanotubes. Hydroxyl groups decorating the surface of the multiwalled carbon nanotubes facilitate intimate contact between the carbon nanotubes and CTF, thus promoting efficient electron transfer. The novel hybrid materials generate CO with a faradaic efficiency up to 81% at an overpotential of 380 mV. The selectivity of the electrocatalysts could be linked to the amount of nitrogen present within the framework.
- This article is part of the themed collection: International Symposium on Green Chemistry 2019


 Please wait while we load your content...
Please wait while we load your content...