Photochemical evolution of hydrogen peroxide on lignins†
Abstract
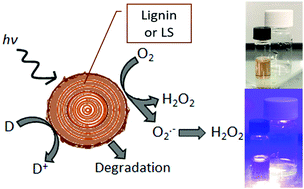
Means of sustainable on-demand hydrogen peroxide production are sought after for numerous industrial, agricultural, and environmental applications. Herein we present the capacity of lignin and lignin sulfonate to behave as photocatalysts that upon irradiation reduce oxygen to hydrogen peroxide. Water-soluble lignin sulfonate acts as a homogeneous photocatalyst in solution, while lignin in thin-film form behaves as a heterogenous photocatalyst. In both cases, the photochemical cycle is closed via the oxidation of electron donors in solution, a process which competes with the autooxidation of lignin. Therefore, lignins can be destructively photooxidized to produce hydrogen peroxide as well as photochemically oxidizing low-oxidation potential species. These findings enable new photochemistry applications with abundant biopolymers and inform the growing body of knowledge on photochemical evolution of hydrogen peroxide.


 Please wait while we load your content...
Please wait while we load your content...