Dissolving used rubber tires†
Abstract
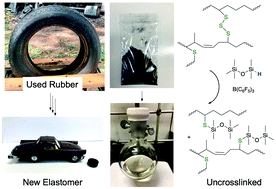
Used automobile tires present an enormous environmental burden. Efficient methods for degradation of the sulfur crosslinks in organic elastomers have proven elusive. We show that the reductive silylation of RS-SR bonds to silyl thio ethers RSSiR′′3 in up to 90% yield using a variety of hydrosilicones occurs in the presence of <1 mol% B(C6F5)3 for model compounds. Sulfur-cured automotive rubber required 10 wt% catalyst for efficient sulfide cleavage. At temperatures ranging from room temperature to 100 °C recoveries of organic polymers as oils from tires using this one step process ranged from 56% for complex mixtures of rubber crumb from ground tires to 93% for butyl rubber (bicycle inner tubes; 87% yield at 100 °C over 30 minutes). After removal of inorganic materials by simple filtration, the recovered polymeric oils were radically or oxidatively crosslinked to generate new elastomers that can be optionally reinforced with the solids recovered in the initial reduction procedure. This mild process constitutes a facile route to reutilize the organic polymers found in automobile and other sulfur-crosslinked rubbers.


 Please wait while we load your content...
Please wait while we load your content...