Halogenated semivolatile acetonitriles as chloramination disinfection by-products in water treatment: a new formation pathway from activated aromatic compounds
Abstract
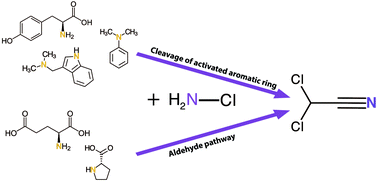
The use of monochloramine as an alternative disinfectant to chlorine in drinking water treatment can lead to increased formation of emerging nitrogenous halogenated disinfection by-products (DBPs), even when the formation of regulated halogenated DBPs has decreased. In this study, we investigated formation of the semivolatile haloacetonitriles (HANs) from model nitrogen-containing compounds (6 amines, 1 amide, 6 amino acids, and 2 nitrogen-containing aromatic chemicals) and natural organic matter (NOM) reference materials after chloramination. In agreement with previous studies, most amino acids formed dichloroacetonitrile (DCAN). Additionally, DCAN formed from two amines containing aromatic rings (N,N-dimethylaniline and 3-(dimethylamino-methyl)indole) and the two nitrogen-containing aromatic chemicals (cotinine and phenytoin). This is the first report of DCAN formation from these precursors. DCAN also formed after chloramination of NOM reference materials, with the highest formation from the NOM material with the highest aromaticity. The results provide new evidence of a DCAN formation pathway from cleavage of activated aromatic structures after electrophilic substitution of chlorine and addition of monochloramine to the ring system. In particular, the results suggest that the previously proposed aldehyde pathway from the amino acid group is not responsible for the majority of DCAN formation from amino acids with an activated aromatic ring system. This newly proposed formation pathway for DCAN from activated aromatic organic matter has significant implications for NOM removal during water treatment to minimise DBP formation. Studies using 15N-labelled monochloramine showed that there was significant incorporation of nitrogen from monochloramine into DCAN, demonstrating that monochloramine disinfection promotes the formation of HANs.
- This article is part of the themed collection: Halogenated (semi)volatile organic compounds (“X(S)VOCs”)


 Please wait while we load your content...
Please wait while we load your content...