Ultra-rapid uptake and the highly stable storage of methane as combustible ice†
Abstract
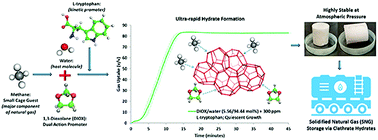
The continuously increasing trend of natural gas (NG) consumption due to its clean nature and abundant availability indicates an inevitable transition to an NG-dominated economy. Solidified natural gas (SNG) storage via combustible ice or clathrate hydrates presents an economically sound prospect, promising high volume density and long-term storage. Herein, we establish 1,3-dioxolane (DIOX) as a highly efficient dual-action (thermodynamic and kinetic promoter) additive for the formation of clathrate (methane sII) hydrate. By synergistically combining a small concentration (300 ppm) of the kinetic promoter L-tryptophan with DIOX, we further demonstrated the ultra-rapid formation of hydrates with a methane uptake of 83.81 (±0.77) volume of gas/volume of hydrate (v/v) within 15 min. To the best of our knowledge, this is the fastest reaction time reported to date for sII hydrates related to SNG technology and represents a 147% increase in the hydrate formation rate compared to the standard water–DIOX system. Mixed methane–DIOX hydrates in pelletized form also exhibited incredible stability when stored at atmospheric pressure and moderate temperature of 268.15 K, thereby showcasing the potential to be industrially applicable for the development of a large-scale NG storage system.


 Please wait while we load your content...
Please wait while we load your content...