Copper-bottomed: electrochemically active bacteria exploit conductive sulphide networks for enhanced electrogeneity†
Abstract
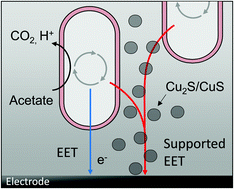
In this study, we demonstrate that anodic electroactive bacteria like Geobacter sulfurreducens generate copper(I) and copper(II) sulphides when grown on copper electrodes. The insoluble copper sulphides form a conductive network within the biofilms, strongly enhancing the biofilm electrogeneity – i.e., the ability of the biofilm to produce electric currents. Compared to biofilms grown on graphite, the average relative current density of copper-based biofilms was 237%, with a maximum geometric current density of 1.59 ± 0.23 mA cm−2. An additional electrochemical CuS deposition prior to biofilm cultivation further increased the bioelectrocatalytic current generation to 2 mA cm−2. The chemical deposition of CuS onto graphite allowed cultivating biofilms with current densities 134% higher than at unmodified graphite. This approach – the chemical CuS deposition onto inexpensive electrode materials – thus represents a promising pathway for the development of scalable, high-performance electrode materials for microbial electrochemical technologies.


 Please wait while we load your content...
Please wait while we load your content...