Insights into the carbon balance for CO2 electroreduction on Cu using gas diffusion electrode reactor designs†
Abstract
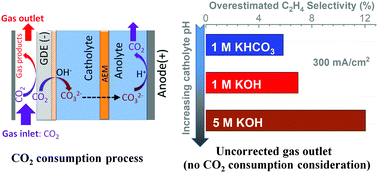
In this work, the carbon balance during high-rate CO2 reduction in flow electrolyzers was rigorously analyzed. The CO2 consumption at gas-diffusion electrodes due to electrochemical conversion and reaction with OH− at the electrode/electrolyte interface leads to a substantial reduction in the volumetric flowrate of gas flow out of the electrolyzer, especially when highly concentrated alkaline electrolytes and elevated current densities are utilized, which is primarily due to an elevated pH at cathode/electrolyte interface. Without considering the CO2 consumption, the faradaic efficiencies for major gas products could be significantly overestimated during high current density CO2 reduction conditions, particularly in the case of high pH electrolyte. In addition, a detailed carbon balance path is elucidated via a two-step procedure of CO2 reaction with OH− at the cathode/electrolyte interface and subsequent CO2 generation at the anode/electrolyte interface caused by a relatively low pH in the vicinity of the anode. Based on the proposed two-step carbon balance path, a systematic exploration of gases released in the anolyte reveals the transformation of a HCO3− or OH− catholyte to a CO32− catholyte, which was further confirmed by pH measurements.


 Please wait while we load your content...
Please wait while we load your content...