Cation-disordered rocksalt transition metal oxides and oxyfluorides for high energy lithium-ion cathodes
Abstract
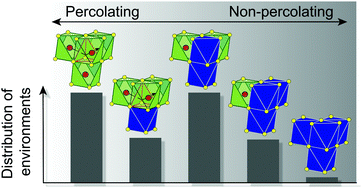
For lithium-ion rechargeable batteries to meet society's ever-growing demands in electrical energy storage, e.g. for the electrification of transportation, for portable electronics and for grid storage applications, novel electrode materials with a large charge storage capacity and a high energy density are needed. Over the last five years, several experimental and theoretical studies have demonstrated the feasibility of disordered rocksalt (DRX) cathodes, that is, lithium transition metal oxide cathodes with a crystalline rocksalt structure but with a disordered arrangement of lithium and transition metal on the cation lattice. We provide here an overview of the current understanding of DRX materials, in terms of their structural and compositional characteristics, as well as their electrochemical properties. We also present important considerations for the design of high performance DRX cathodes and suggest future research directions. Because no specific order is needed, DRX compounds can be composed of a wide variety of transition metal species, which can create long-term benefits for the lithium battery industry by making it less reliant on scarce and expensive raw materials. While some DRX compositions can simply be synthesized at high temperature to induce thermal cation disorder, other compositions require mechanochemical methods to induce a disordered arrangement of cation species. Cation disorder leads to unique lithium transport properties, small volume changes during charge–discharge cycling and sloping electrochemical profiles. Fluorine substitution for oxygen and the incorporation of high-valent d0 transition metals in the bulk DRX structure are two strategies used to increase the lithium content in the material, improve lithium percolation and to keep the valence of redox-active metal species low so that high transition metal redox capacity can be obtained. Short-range cation order, which is affected by thermal treatment, metal composition and fluorine substitution, has a significant impact on electrochemical performance. Moreover, fluorine substitution for oxygen improves long-term capacity retention by significantly reducing anion-based charge compensation mechanisms during charge. Fluorinated DRXs have recently demonstrated reversible capacities >300 mA h g−1 and extremely high energy densities approaching 1000 W h kg−1, holding promise for a nearly two-fold increase in the energy density of commercial lithium-ion batteries.
- This article is part of the themed collection: 2024 Journal of Materials Chemistry Lectureship winner: Raphaële Clément


 Please wait while we load your content...
Please wait while we load your content...