Strain stabilized nickel hydroxide nanoribbons for efficient water splitting†
Abstract
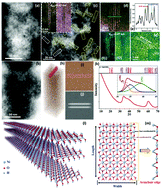
Development of efficient and durable oxygen evolution reaction (OER) catalysts has a direct impact on the water splitting efficiency and cost effectiveness. In this work, we report the successful synthesis of a new Ni(OH)2 structure, strain-stabilized Ni(OH)2 nanoribbons (NR-Ni(OH)2) two to three layers thick, with widths of 2–5 nm, via an electro-oxidation route. Conventional Ni(OH)2 usually has negligible OER activity, while NR-Ni(OH)2 shows high activity for the oxygen evolution reaction and an overpotential of 162 millivolts and furthermore exhibits long-term stability in alkaline electrolyte. The substantial reduction in the overpotential of NR-Ni(OH)2 is due to its easier OOH* adsorption by the active four-coordinated Ni edge sites. The enhanced catalytic activity of NR-Ni(OH)2 makes it an excellent candidate for industrial applications.


 Please wait while we load your content...
Please wait while we load your content...