Low-spin and spin-crossover iron(ii) complexes with pyridyl-benzimidazole ligands: synthesis, and structural, magnetic and solution study†
Abstract
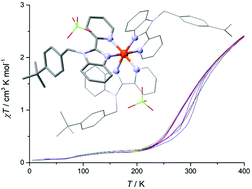
Two tridentate ligands (L1 = 2,6-bis(1-(3,5-di-tert-butylbenzyl)-1H-benzimidazol-2-yl)pyridine and L2 = 2,6-bis(1-(4-tert-butylbenzyl)-1H-benzimidazol-2-yl)pyridine) and one didentate ligand (L3 = 1-(4-tert-butylbenzyl)-2-pyridine-2-yl-1H-benzimidazol) were used for the synthesis of eight mononuclear Fe(II) compounds 1–8 containing miscellaneous counterions. Single-crystal X-ray diffraction analysis confirmed the expected molecular structures of all the reported coordination compounds and revealed the octahedral geometry of metal centres in the complex dications of 1–8. Compounds 1–6 prepared from tridentate ligands were low-spin and, therefore, diamagnetic up to 400 K. On the other hand, compounds 7 and 8, in which the Fe(II) centre was coordinated with didentate ligand L3, exhibited temperature and light triggered spin-crossover behaviour. The theoretical calculations supported the experimental magnetic investigation and helped to explain the electronic structures of the reported complexes with respect to the occurrence of thermal and light induced spin state switching. In addition, the solution redox properties of compounds 1–8 were investigated by cyclic voltammetry.


 Please wait while we load your content...
Please wait while we load your content...