Long-range coupling in cyclic silanes†
Abstract
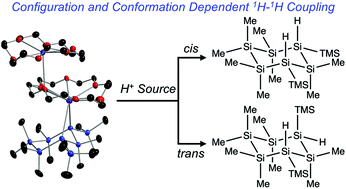
We report the synthesis of a mixed methyl- and hydro-substituted cyclosilane (1) possessing cis/trans stereoisomerism. Each diastereomer of 1 possesses distinct symmetry elements (cis-1: Cs-symmetric; trans-1: C2-symmetric). Cyclosilane 1 is a model system to probe configuration- and conformation-dependent long-range proton–proton coupling. Extensive NMR spectroscopic characterization is reported, including one-dimensional 1H NMR and 29Si DEPT and INEPT+ spectra and two-dimensional 1H–29Si and 1H–1H correlated spectroscopy (HSQC, HMBC, COSY). On the basis of these experiments, molecular connectivity consistent with four-bond 1H–1H coupling is confirmed.


 Please wait while we load your content...
Please wait while we load your content...
