Al(iii)-NTA-fluoride: a simple model system for Al–F binding with interesting thermodynamics†
Abstract
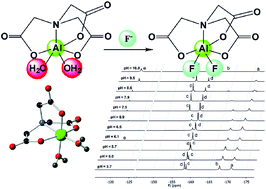
Al(III) complexes are extensively studied as [18F]fluoride carriers in positron emission tomography. However, our limited knowledge on their thermodynamic and kinetic properties has hindered efforts to easily prepare radiochemically pure compounds while simultaneously reducing the overall labeling time. Thus, to improve our understanding of fluoride binding to coordinatively unsaturated Al(III) complexes, we investigated the ternary system Al(III)–H3NTA–F− (H3NTA = nitrilo-triacetic acid) by NMR, potentiometry and X-ray diffraction. Our results show that the [Al(NTA)] complex binds two water molecules, which are replaced by fluorides. Individual species and isomers show separate 19F NMR signals and different stability constants. The two available positions on the [Al(NTA)] complex feature significantly different properties in terms of basicity of the coordinated water molecules and preferential binding of fluoride anions. Fluorides are effectively bound in weakly acidic or neutral solutions, whereas hydroxido species are preferentially formed in alkaline solutions. Our experimental observations were rationalized by theoretical calculations: predictions of the energy ordering of complexes and isomers, interpretation of 19F NMR chemical shifts, and natural bonding orbital analysis. Radiolabeling of [Al(NTA)] with [18F]fluoride gave low yields that confirmed a high affinity of the complex for hydroxide anions.


 Please wait while we load your content...
Please wait while we load your content...