Synthesis and antiproliferative activity of hindered, chiral 1,2-diaminodiamantane platinum(ii) complexes†
Abstract
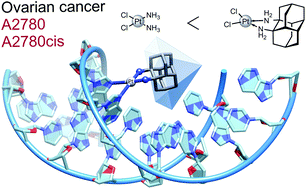
Platinum-based antineoplastic agents play a major role in the treatment of numerous types of cancer. A new bulky, lipophilic, and chiral ligand based on 1,2-diaminodiamantane in both of its enantiomeric forms was employed for the preparation of new platinum(II) complexes with chloride and oxalate ligands. The dichloride complexes have a higher solubility and were evaluated as anti-proliferation agents for human ovarian cancer cell lines A2780 and cisplatin-resistant A2780cis. Its R,R-enantiomer showed increased efficacy compared to cisplatin for both cancer cell lines. A chromatographic approach was used to estimate the solvent partition coefficient of the dichloride complex. The binding of diamondoid-based platinum complexes to nucleotides was tested for both enantiomers with guanosine monophosphate (GMP) and deoxyguanosine monophosphate (dGMP) and occurs at a similar or faster rate for both isomers compared to cisplatin despite greatly increased steric demand. These findings highlight the potential in 1,2-diaminodiamantane as a viable pharmacophore.


 Please wait while we load your content...
Please wait while we load your content...