Intermediates and products of the reaction of Zn(ii) organyls with tetrel element Zintl ions: cluster extension versus complexation†
Abstract
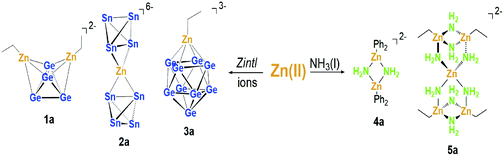
The discovery of low-valent Zn compounds resulted in the renaissance of organometallic Zn complexes. Polyhedral clusters of tetrel elements can interact with Zn atoms either as Lewis donors or by incorporation of the Zn atoms as additional cluster vertices. Herein we report the reactions of ZnR2 (R = ethyl (Et), pentamethylcyclopentadiene (Cp*), phenyl (Ph) and 1,3,5-trimethylbenzene (Mes)) with Zintl ions of the types [E4]4− (E = Ge, Sn) and [Ge9]4− in liquid ammonia. Besides the desired Zintl ion complexes, intermediates were isolated that give insight into the reaction of organo Zn compounds in liquid ammonia. Three ions, [(η3:η3-Ge4)(ZnEt)2]2− (1a), [(η2-Sn4)Zn(η2-Sn4)]6− (2a) and [(η4-Ge9)(ZnEt)]3− (3a), were obtained and characterized by means of single crystal X-ray diffraction analysis. Furthermore, amides [(ZnPh2)2(μ2-NH2)2]2− (4a) and {[Zn(μ2-NH2)4][(ZnEt)2(μ2-NH2)2]2}2− (5a) were formed during the reactions, together with the addition products [ZnPh3]− (6a) and [ZnMes3]− (7a) and an anion [Cp*]− (8a), suggesting the following reaction sequence: in liquid ammonia the Zintl anions form amides, which then serve as ligands for ZnR2 molecules. The NH2− ligands weaken the corresponding Zn–R bonds, and thus bond cleavage and the addition of the Zintl anion to the Zn ion can take place, additionally promoted by the trapping of the leaving group R− by unreacted Zn organyls with the formation of [ZnR3]−.


 Please wait while we load your content...
Please wait while we load your content...