A dinuclear rhenium complex in the electrochemically driven homogeneous and heterogeneous H+/CO2-reduction†
Abstract
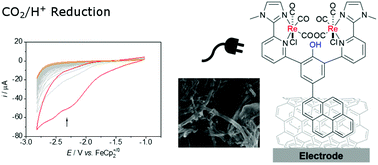
A dinculear Re(CO)3 complex with a proton responsive phenol unit and a pyrene anchor in the ligand backbone was investigated in the electrochemical CO2/H+ conversion in solution and adsorbed on multi walled carbon nanotubes (MWCNT) on an GC electrode surface. The pyrene group unit is introduced at the end of the ligand synthesis via a coupling reaction, which allows for a versatile ligand modification in order to tune the electronic properties or to introduce various anchor groups for heterogenisation at a late stage. The redox chemistry of the pyrene-α-diimine-Re(CO)3 complex, 1, was investigated in N,N-dimethylformamide (dmf), including IR-spectroelectrochemical (IR-SEC) characterisation of the short lived, reduced species. Subsequently, the electrochemical H+/CO2-reduction catalysis in dmf/water was investigated. The complex catalyses syngas formation yielding CO and H2 with similar rates, namely in Faraday yields of 45% and 35%, respectively. Since the similar complex without the pyrene anchor in the backbone, I, prefers CO2 over H+ reduction, the formation of syngas was rationalised by the small differences in the redox properties and pKa values of the phenol-pyrene unit in regard to phenol unit as in I. Subsequently, the complex was adsorbed on multi walled carbon nanotubes (MWCNT) on a GC electrode surface. Scanning electron microscopy (SEM) and X-ray photoelectron spectroscopy (XPS) confirmed coating of the electrode. The immobilised complex was utilised in the electrochemical CO2/H+ reduction in dmf/water, however, the complex quickly desorbed under reductive conditions, likely due to the good solubility of the reduced species. Water as a solvent prevents desorption as confirmed by XPS, however, then a preference for H2 formation over syngas formation was observed under electrocatalytic conditions. Thus, these experiments show, that the results obtained in aqueous organic solution are not easily transferable to the heterogeneous systems operating in water due to changes in the reaction rates for competing pathways.


 Please wait while we load your content...
Please wait while we load your content...