Mechanistic diversity in acetophenone transfer hydrogenation catalyzed by ruthenium iminophosphonamide complexes†
Abstract
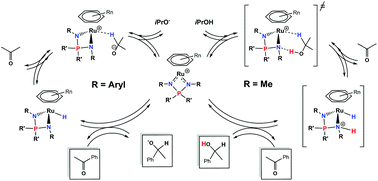
A series of arene ruthenium iminophosphonamide complexes, [(Arene)RuCl{R2P(NR′)2}] (1), bearing various arenes and R,R′ substituents on the NPN ligand have been investigated as precatalysts in acetophenone transfer hydrogenation in basic and base-free isopropanol. The results clearly demonstrate the presence of two distinct reaction mechanisms, which are controlled by the basicity of the N-atoms. Complexes 1 in which both R′ substituents are aryl groups are only active once the neutral hydride complex [(Arene)RuH{R2P(NR′)2}] (2) is generated in basic isopropanol, the latter being able to reduce a ketone via a stepwise hydride and proton transfer. On the other hand, complexes in which at least one R′ group is Me readily catalyze the reaction in the absence of base. In the latter case, the results of kinetic studies and DFT calculations support an outer-sphere concerted asynchronous hydride and proton transfer assisted by the basic N-atom of the NPN ligand, which promotes catalysis via precoordination of an alcohol molecule by hydrogen bonding.


 Please wait while we load your content...
Please wait while we load your content...