Spin modification of iron(ii) complexes via covalent (dative) and dispersion guided non-covalent bonding with N-heterocyclic carbenes: DFT, DLPNO-CCSD(T) and MCSCF studies†
Abstract
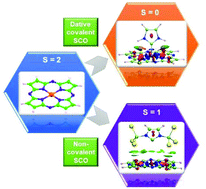
This paper reports a systematic quantum chemical study of the complexation of iron(II) phthalocyanine (FePc) and Fe(II)–porphyrazine (FePz) complexes with N-heterocyclic carbenes. The complex can be stabilised either in a singlet or in a triplet state depending on the choice of substituents in the same carbene core. Here, the spin crossover is invoked either via direct bonding or via a dispersion guided non-covalent interaction. Stabilization of intermediate spin of Fe(II) phthalocyanine/Fe(II)–porphyrazine type systems via complexation with carbenes is hitherto unknown. For calculations we have considered a wide range of density functional theory (DFT) levels, including GGA, hybrid and double hybrid functionals. The results obtained using DFT are further supported by domain-based local pair natural orbital-CCSD(T) (DLPNO-CCSD(T)) and multi-configurational self-consistent field (MCSCF) results.


 Please wait while we load your content...
Please wait while we load your content...