Towards dual SPECT/optical bioimaging with a mitochondrial targeting, 99mTc(i) radiolabelled 1,8-naphthalimide conjugate†
Abstract
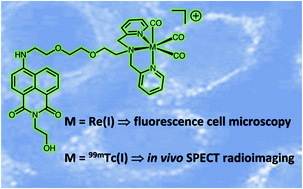
A series of six different 1,8-naphthalimide conjugated dipicolylamine ligands (L1–6) have been synthesised and characterised. The ligands possess a range of different linker units between the napthalimide fluorophore and dipcolylamine chelator which allow the overall lipophilicity to be tuned. A corresponding series of Re(I) complexes have been synthesised of the form fac-[Re(CO)3(L1–6)]BF4. The absorption and luminescence properties of the ligands and Re(I) complexes were dominated by the intramolecular charge transfer character of the substituted fluorophore (typically absorption ca. 425 nm and emission ca. 520 nm). Photophysical assessments show that some of the variants are moderately bright. Radiolabelling experiments using a water soluble ligand variant (L5) were successfully undertaken and optimised with fac-[99mTc(CO)3(H2O)3]+. Confocal fluorescence microscopy showed that fac-[Re(CO)3(L5)]+ localises in the mitochondria of MCF-7 cells. SPECT/CT imaging experiments on naïve mice showed that fac-[99mTc(CO)3(L5)]+ has a relatively high stability in vivo but did not show any cardiac uptake, demonstrating rapid clearance, predominantly via the biliary system along with a moderate amount cleared renally.


 Please wait while we load your content...
Please wait while we load your content...