A low temperature pyrolytic route to amorphous quasi-hexagonal boron nitride from hydrogen rich (NH4)3Mg(BH4)5†‡
Abstract
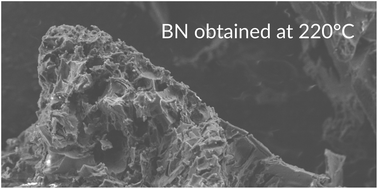
Pure amorphous quasi-hexagonal boron nitride with minute amounts of amorphous quasi-cubic form was obtained via thermal decomposition of a novel tri-ammonium magnesium penta-borohydride precursor, (NH4)3Mg(BH4)5, in the temperature range of 220–250 °C, which is significantly lower than 1000–1500 °C applied in industrial approaches. The (NH4)3Mg(BH4)5 precursor, the most hydrogen rich mixed-cation borohydride salt known to date (21 wt% H), was prepared via low temperature high-energy dry disc-milling. The compound adopts a tetragonal I4/mcm unit cell isostructural with Rb3Mg(BH4)5 and Cs3Mg(BH4)5. The multi-step thermal decomposition yields hydrogen contaminated with B2H6 and borazine volatiles. The solid residue rinsed with water corresponds to amorphous boron nitride of high purity as evidenced by 11B MAS NMR, PXRD, FTIR and EDX analyses.


 Please wait while we load your content...
Please wait while we load your content...