Abstract
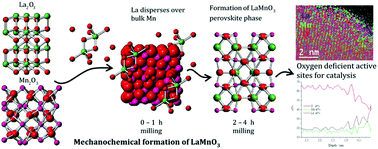
Mechanochemistry offers a solventless, ‘waste free’ route to preparing metal oxide catalysts, however, there is limited information on the chemical steps involved. In this work, the perovskite LaMnO3 has been successfully synthesized via mechanochemistry from metal oxide powders, La2O3 and Mn2O3, at room temperature, using a planetary ball mill. Separate ex situ ‘time slices’ were taken during the milling procedure to provide insights into the underlying chemistry. The crystalline material was assessed using XRD, which identified 100% perovskite phase after 3 h of milling. Conversely, characterization by X-ray absorption spectroscopy (XAS) at both the Mn K-edge and La L3-edge provides a very different picture. The XAS data shows that there are significant structural alterations as early as 30 min of milling, with the La precursor dispersed over Mn2O3. Increasing milling time then allows for mechanical activation of both precursors and the formation of powdered LaMnO3, with no calcination step required. The XAS highlights that there is a significant amount of amorphous, oxygen deficient, content even when XRD has identified 100% perovskite phase. The samples were tested for the decomposition of the environmental pollutant N2O; at a milling time of 3 h, the LaMnO3 catalyst displays a much early onset production of N2 compared to a traditional sol–gel synthesized LaMnO3, resulting from increased oxygen deficiency at the surface, confirmed by XPS and STEM-EELS. This is an encouraging sign that mechanochemical routes can be harnessed to provide a sustainable route to preparing mixed metal oxide catalysts with enhanced catalytic performance.


 Please wait while we load your content...
Please wait while we load your content...