The direct synthesis of hydrogen peroxide from H2 and O2 using Pd–Ga and Pd–In catalysts†
Abstract
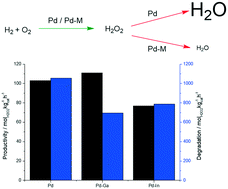
The direct synthesis of hydrogen peroxide is investigated using PdGa/TiO2 and PdIn/TiO2 catalysts prepared by an acid-washed sol-immobilisation procedure, which allows for good control of particle size. The introduction of both Ga and In into a supported Pd catalyst leads to significantly reduced rates of H2O2 degradation in comparison to the monometallic counterpart. Detailed characterisation reveals that this enhancement is a result of selectively tuning the ratio of Pd oxidation states.


 Please wait while we load your content...
Please wait while we load your content...