Investigation of the stability of NiFe-(oxy)hydroxide anodes in alkaline water electrolysis under industrially relevant conditions†
Abstract
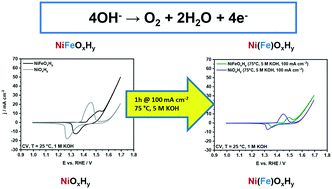
NiFe-(Oxy)hydroxide is one of the most active electrocatalysts for the oxygen evolution reaction (OER) in alkaline conditions. Herein we investigated the stability of NiFe-(oxy)hydroxide anodes at high current densities (100 mA cm−2) at different temperatures (25, 75 °C) and base concentrations (1, 5, 10 M KOH). While polarization led to minor structural and compositional changes under standard conditions (25 °C, 1 M KOH), the anodes were severely impacted at higher temperature (75 °C) and base concentrations (5, 10 M KOH). Overall leaching and preferential leaching of Fe (resulting in a lower Fe/Ni ratio) led to decreased OER performance and increased charge transfer resistance for the samples tested at industrially relevant conditions. A dramatic loss in the catalytic activity occurred for the sample polarized at 75 °C in 10 M KOH: besides extensive leaching, a transformation of Ni(OH)2 into NiO was noted in this case. For pure NiOxHy, incorporation of Fe impurities from the electrolyte during polarization at 75 °C in 5 M KOH led to an improvement in the catalytic activity and charge-transfer properties, approaching the performance of NiFeOxHy.


 Please wait while we load your content...
Please wait while we load your content...