Aerobic oxidation of 1,6-hexanediol to adipic acid over Au-based catalysts: the role of basic supports
Abstract
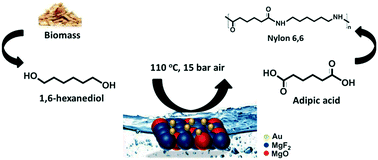
1,6-Hexanediol is one of the most relevant building blocks originating from biomass and it transforms into adipic acid for polymer synthesis. Herein, we examine the selective oxidation of 1,6-hexanediol to adipic acid over Au-based catalysts, in the aqueous phase, under base-free conditions. Particularly, the absence of a base allows the neutralization step at the end of the reaction to be avoided. The influences of various supports (MgO, BaO, NiO, and TiO2) and substrate/gold molar ratios were studied. Under the conditions used, the leaching of Mg in the mixture of MgF2 and MgO was limited by diluting the basic sites in the support. The highest selectivity to adipic acid (43%) was achieved at 110 °C under 15 bar of air in the presence of 2 wt% Au/0.6MgF2–0.4MgO.


 Please wait while we load your content...
Please wait while we load your content...