Heteroatom-participated lignin cleavage to functionalized aromatics
Abstract
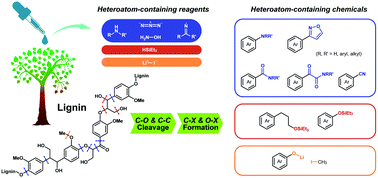
Lignin, the most abundant aromatic polymer in nature, enables sustainable supply of miscellaneous aromatics as green fuels and chemicals. Obtaining the value-added aromatics from lignin, though subjected to enormous research efforts, mainly relies on depolymerization induced by activated hydrogen species or oxygen species, delivering hydrocarbons and oxygenates. The future bio-refinery demands a broad spectrum of fine chemicals, especially those containing elements other than C, H and O. Heteroatom-containing compounds have emerged as powerful reagents to participate in the bond cleavage in lignin; meanwhile, the obtained heteroatom-containing aromatics, which could be used as dye precursors, pharmaceutical precursors, hydrogen storage materials, etc., extend the application of lignin-derived products. This tutorial review updates recent advances in the lignin C–C and C–O bond cleavages induced by heteroatoms X (N, Si, I and Li), which also lead to functionalized products containing C–X and O–X bonds. The representative reaction pathways and feasibilities in lignin models and extracts are summarized. Potential applications of functionalized monomers in synthetic transformations, pharmaceuticals, dyes and energy storage are also discussed.
- This article is part of the themed collection: Catalytic advances for biomass conversion and upgrading


 Please wait while we load your content...
Please wait while we load your content...