Halogen bonds of halonium ions
Abstract
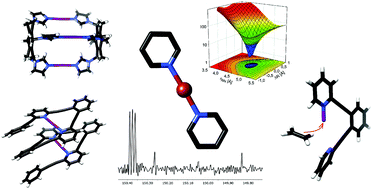
Due to their electron deficiency, halonium ions act as particularly strong halogen bond donors. By accepting electrons in both lobes of their empty p-orbital, they are capable of simultaneously interacting with two Lewis bases. The interaction presumes the formation of three molecular orbitals and is accordingly typically entitled as a three-center halogen bond. In analogy to the [D–H–D]+ hydrogen bonds, which are at times entitled as short and strong bonds, the [D–X–D]+ halogen bonds of halonium ions show Bondi normalized interatomic distances of 0.6–0.7 and possess both charge transfer and electrostatic characteristics. The three-center halogen bond of halonium ions shows distinct differences in its properties from coordinative bonds of transition metals and is therefore applicable as a complementary synthon in supramolecular chemistry. The three-center halogen bond modulates the reactivity of halonium ions and is hence a useful tool for synthetic organic chemistry. Following the discussion of the nature and properties of halonium ions’ halogen bonds, this tutorial review provides an overview of their current applications to stimulate future developments.


 Please wait while we load your content...
Please wait while we load your content...