Transition-metal mediated carbon–sulfur bond activation and transformations: an update
Abstract
Carbon–sulfur bond cross-coupling has become more and more attractive as an alternative protocol to establish carbon–carbon and carbon–heteroatom bonds. Diverse transformations through transition-metal-catalyzed C–S bond activation and cleavage have recently been developed. This review summarizes the advances in transition-metal-catalyzed cross-coupling via carbon–sulfur bond activation and cleavage since late 2012 as an update of the critical review on the same topic published in early 2013 (Chem. Soc. Rev., 2013, 42, 599–621), which is presented by the categories of organosulfur compounds, that is, thioesters, thioethers including heteroaryl, aryl, vinyl, alkyl, and alkynyl sulfides, ketene dithioacetals, sulfoxides including DMSO, sulfones, sulfonyl chlorides, sulfinates, thiocyanates, sulfonium salts, sulfonyl hydrazides, sulfonates, thiophene-based compounds, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S functionality-bearing compounds such as thioureas, thioamides, and carbon disulfide, as well as the mechanistic insights. An overview of C–S bond cleavage reactions with stoichiometric transition-metal reagents is briefly given. Theoretical studies on the reactivity of carbon–sulfur bonds by DFT calculations are also discussed.
S functionality-bearing compounds such as thioureas, thioamides, and carbon disulfide, as well as the mechanistic insights. An overview of C–S bond cleavage reactions with stoichiometric transition-metal reagents is briefly given. Theoretical studies on the reactivity of carbon–sulfur bonds by DFT calculations are also discussed.
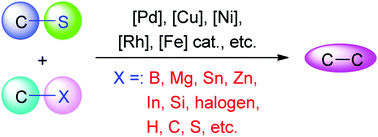


 Please wait while we load your content...
Please wait while we load your content...