Intermolecular radical carboamination of alkenes
Abstract
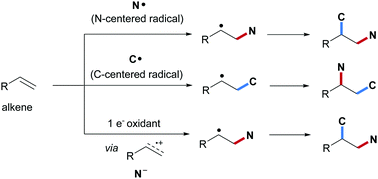
Vicinal alkene carboamination is a highly efficient and practical synthetic strategy for the straightforward preparation of diverse and valuable amine derivatives starting from simple compounds. During the last decade that approach has found continuous research interests and various practical methods have been developed using transition-metal catalysis. Driven by the renaissance of synthetic radical chemistry, intermolecular radical alkene carboamination comprising a C–C bond and a C–N bond forming step has been intensively investigated recently culminating in novel strategies and improved protocols which complement existing methodologies. Radical alkene carboamination can be achieved via three different reaction modes. Such cascades can proceed through N-radical addition to an alkene with subsequent C–C bond formation leading to 2,1-carboamination products. Alternatively, the C–C bond can be installed prior to the C–N bond via initial C-radical addition to the alkene with subsequent β-amination resulting in 1,2-carboamination. The third mode comprises initial single electron oxidation of the alkene to the corresponding alkene radical cation that gets trapped by an N-nucleophile and the cascade is terminated by radical C–C bond formation. In this review, the three different conceptual approaches will be discussed and examples from the recent literature will be presented. Further, the reader will get insights into the mechanism of the different transformations.


 Please wait while we load your content...
Please wait while we load your content...