Tuning the miscibility of water in imide-based ionic liquids†
Abstract
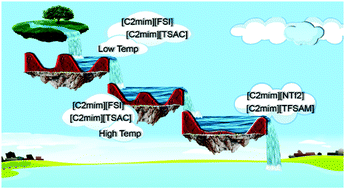
Liquid–liquid phase behavior measurements were performed for binary mixtures of water and ionic liquids (ILs) containing the same 1-ethyl-3-methylimidazolium ([C2mim]+) cation and different imide-based anions, having symmetric (bis(fluorosulfonyl)imide ([FSI]−)) or asymmetric structures (2,2,2-trifluoromethylsulfonyl-N-cyanoamide ([TFSAM]−) and 2,2,2-trifluoro-N-(trifluoromethylsulfonyl)acetamide ([TSAC]−)). An inversion of phase behavior was observed: while below ∼298 K, the miscibility of water in the studied ILs increases according to the order [C2mim][TSAC] < [C2mim][FSI] < [C2mim][NTf2], for temperatures above ∼303 K, the reverse trend is observed [C2mim][NTf2] < [C2mim][FSI] < [C2mim][TSAC]. In turn, above ∼306 K the [C2mim][TFSAM] is completely miscible with H2O in all ranges of concentrations. The obtained results also revealed an unusual water solubility variation of 11% in [C2mim][FSI], and 20% in [C2mim][TSAC], when the system temperature was changed by less than 1 K, around 298 K and 301 K, respectively. Molecular Dynamics (MD) simulations were used to understand the IL–water interactions and rationalize the experimental observations. These results suggested that the miscibility trends are mainly related to the ability of the water molecules to form water–anion and water–water aggregates in solution.


 Please wait while we load your content...
Please wait while we load your content...