A three-point coarse-grained model of five-water cluster with permanent dipoles and quadrupoles
Abstract
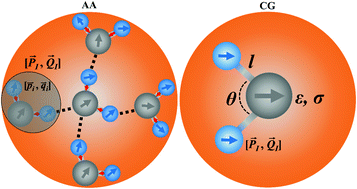
As coarse-grained (CG) studies of large biomolecules increase, developments of reliable CG solvent models become particularly important. In this work, we reduce five water molecules into a three-point CG model with permanent dipole and quadrupole moments. In the CG force field, the modified Morse potential is utilized and an ideal three-water cluster is designed to derive CG-level permanent multipoles. The new CG model is parametrized on the AMOEBA polarizable force field. Various important properties of liquid water are examined to validate the new CG model. Results show that the new CG model can correctly reproduce certain important experimental properties such as density, isothermal compressibility and relative static dielectric permittivity, even better than the existing AA models. Additionally, the CPU tests reveal that the CG model can accelerate molecular dynamics simulations by a factor of 19 compared to the popular AA force field. Compared with the fix-point-charge model widely used in other CG models, the permanent-multipole-based CG model describes more rigid electrostatic attractions. This study also illustrates that the permanent multipole moments contribute a lot to the electrostatic calculations in CG simulation.


 Please wait while we load your content...
Please wait while we load your content...