1D materials from ionic self-assembly in mixtures containing chromonic liquid crystal mesogens†
Abstract
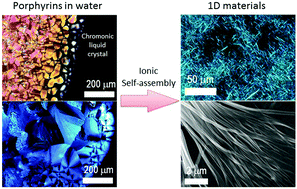
Ionic self-assembly is a simple yet powerful method to obtain robust nanostructures. Herewith, we use mixtures of oppositely-charged porphyrins that can act as mesogens to form chromonic liquid crystals in water, i.e., molecular stacks with orientational (nematic) or positional (hexagonal) order. Electrostatic locking coupled with π–π interactions between aromatic groups within the stacks, together with inter-stack hydrogen bonding induce formation of all-organic crystalline nanofibers with high aspect ratio (a few tenths of nanometers in width but several tenths of micrometers in length) and that display branching. The nanofibers prepared from metal-free porphyrin units feature interesting optical properties, including an absorption spectrum that is different from the simple sum of the individual spectra of the components, which is attributed to a striking aggregation-induced chromism. When in contact with some polar organic solvents the materials become fluorescent, as a result of disaggregation. In a proof-of-concept, the obtained self-assembled one-dimensional (1D) materials were carbonized (yield ca. 60%) to produce nitrogen-doped carbon nanofibers that can be used as active electrode materials for energy storage applications.


 Please wait while we load your content...
Please wait while we load your content...