Isomer-selected ion–molecule reactions of acetylene cations with propyne and allene†
Abstract
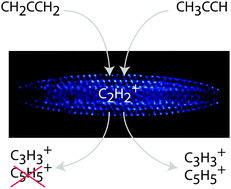
One of the fundamental goals of chemistry is to determine how molecular structure influences interactions and leads to different reaction products. Studies of isomer-selected and resolved chemical reactions can shed light directly on how form leads to function. In the following, we present the results of gas-phase reactions between acetylene cations (C2D2+) with two different isomers of C3H4: propyne (DC3D3) and allene (H2C3H2). Our highly controlled, trapped-ion environment allows for precise determination of reaction products and kinetics. From these results, we can infer details of the underlying reaction dynamics of C2H2+ + C3H4. Through the synergy of experimental results and high-level quantum chemical potential energy surface calculations, we are able to identify distinct reaction mechanisms for the two isomers. We find long-range charge exchange with no complex formation is favored for allene, whereas charge exchange leads to an intermediate reaction complex for propyne and thus, different products. Therefore, this reaction displays a pronounced isomer-selective bi-molecular reactive process.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...