Study of interactions between Brønsted acids and triethylphosphine oxide in solution by 31P NMR: evidence for 2 : 1 species†
Abstract
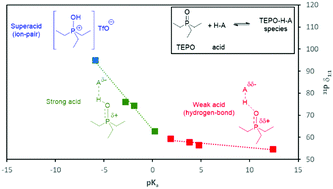
The variation of the 31P chemical shift of triethylphosphine oxide in CDCl3 solution with a series of Brønsted acids at different molar ratios allows the determination of the value for the 1 : 1 species (δ1 : 1), which is much lower than the reported value at infinite dilution. This value correlates with the pKa of the acid in two zones, for acids stronger and weaker than TEPO–H+. The acid strength also controls the exchange rate in solution. The evolution of the chemical shift at high acid/TEPO molar ratios indicates the existence of a second TEPO–acid interaction, which is also dependent on the acid strength. This interaction is much more favorable in the case of a diacid, which shows chemical shift higher than expected for its pKa1 value.


 Please wait while we load your content...
Please wait while we load your content...