Optical and dielectric properties of lead perovskite and iodoplumbate complexes: an ab initio study†
Abstract
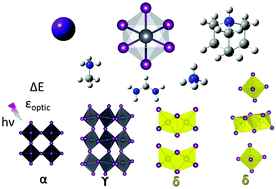
Organic–inorganic lead halide perovskites (APbI3) have shown tremendous growth in solar cell efficiencies; however, our atomistic understanding of the intrinsic instability due to A-cation size effects is far from satisfactory. Here, we report a dispersion corrected density functional theory study of the influence of the A-cation size on the lattice structures of perovskite (α-cubic and γ-orthorhombic) and non-perovskite phases (δ-hexagonal and δ-orthorhombic) through isotropic (cesium and ammonium) and anisotropic cations (methylammonium, formamidinium and dabconium). We found that the local coordination environmental changes and noncovalent interactions are crucial in explaining the enthalpy of formation and relative energy stability. The subtler A-cation's influence on electronic polarization of perovskite and non-perovskite structures were estimated through the optical dielectric constant and then compared with experimental values.


 Please wait while we load your content...
Please wait while we load your content...