Selective bond breaking of halothane induced by electron transfer in potassium collisions
Abstract
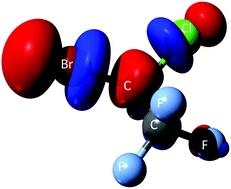
We present novel experimental results of negative ion formation of halothane (C2HBrClF3) upon electron transfer from hyperthermal neutral potassium atoms (K°) in the collision energy range of 8–1000 eV. The experiments were performed in a crossed molecular beam setup allowing a comprehensive analysis of the time-of-flight (TOF) mass negative ions fragmentation pattern and a detailed knowledge of the collision dynamics in the energy range investigated. Such TOF mass spectra data show that the only negative ions formed are Br−, Cl− and F−, with a strong energy dependence in the low-energy collision region, with the bromine anion being the most abundant and sole fragment at the lowest collision energy probed. In addition, potassium cation (K+) energy loss spectra in the forward scattering direction were obtained in a hemispherical energy analyser at different K° impact energies. In order to support our experimental findings, ab initio quantum chemical calculations have been performed to help interpret the role of the electronic structure of halothane. Potential energy curves were obtained along the C–X (X = Br, Cl) coordinate to lend support to the dissociation processes yielding anion formation.


 Please wait while we load your content...
Please wait while we load your content...