Homogeneous nucleation of carbon dioxide in supersonic nozzles I: experiments and classical theories†
Abstract
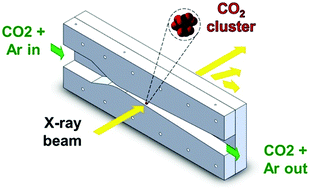
We studied the homogeneous nucleation of carbon dioxide in the carrier gas argon for concentrations of CO2 ranging from 2 to 39 mole percent using three experimental methods. Position-resolved pressure trace measurements (PTM) determined that the onset of nucleation occurred at temperatures between 75 and 92 K with corresponding CO2 partial pressures of 39 to 793 Pa. Small angle X-ray scattering (SAXS) measurements provided particle size distributions and aerosol number densities. Number densities of approximately 1012 cm−3, and characteristic times ranging from 6 to 13 μs, resulted in measured nucleation rates on the order of 5 × 1017 cm−3 s−1, values that are consistent with other nucleation rate measurements in supersonic nozzles. Finally, we used Fourier transform infrared (FTIR) spectroscopy to identify that the condensed CO2 particles were crystalline cubic solids with either sharp or rounded corners. Molecular dynamics simulations, however, suggest that CO2 forms liquid-like critical clusters before transitioning to the solid phase. Furthermore, the critical clusters are not in thermal equilibrium with the carrier gas. Comparisons with nucleation theories were therefore made assuming liquid-like critical clusters and incorporating non-isothermal correction factors.


 Please wait while we load your content...
Please wait while we load your content...